
#SILICON ELECTRON CONFIGURATION FULL#
To find its noble gas shorthand, look at the full electron configuration and see how many filled shells there are. Select all answers that apply Mercury (Hg) Silicon(Si) Xenon (xe). So each s subshell has one orbital, each p subshell has three orbitals, each d subshell has five orbitals, and each f subshell has seven orbitals.Īs mentioned above, the electron configuration of silicon is 1s 2 2s 2 2p 6 3s 2 3p 2. The full electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2. The full or longhand electron configuration involves going from the 1s orbital and to. Where, ℓ = azimuthal quantum number of the subshellįor s subshell, ℓ = 0 For p subshell, ℓ = 1 For d subshell, ℓ = 2 For f subshell, ℓ = 3 We can calculate the number of orbitals in each subshell using the formula: 2ℓ + 1
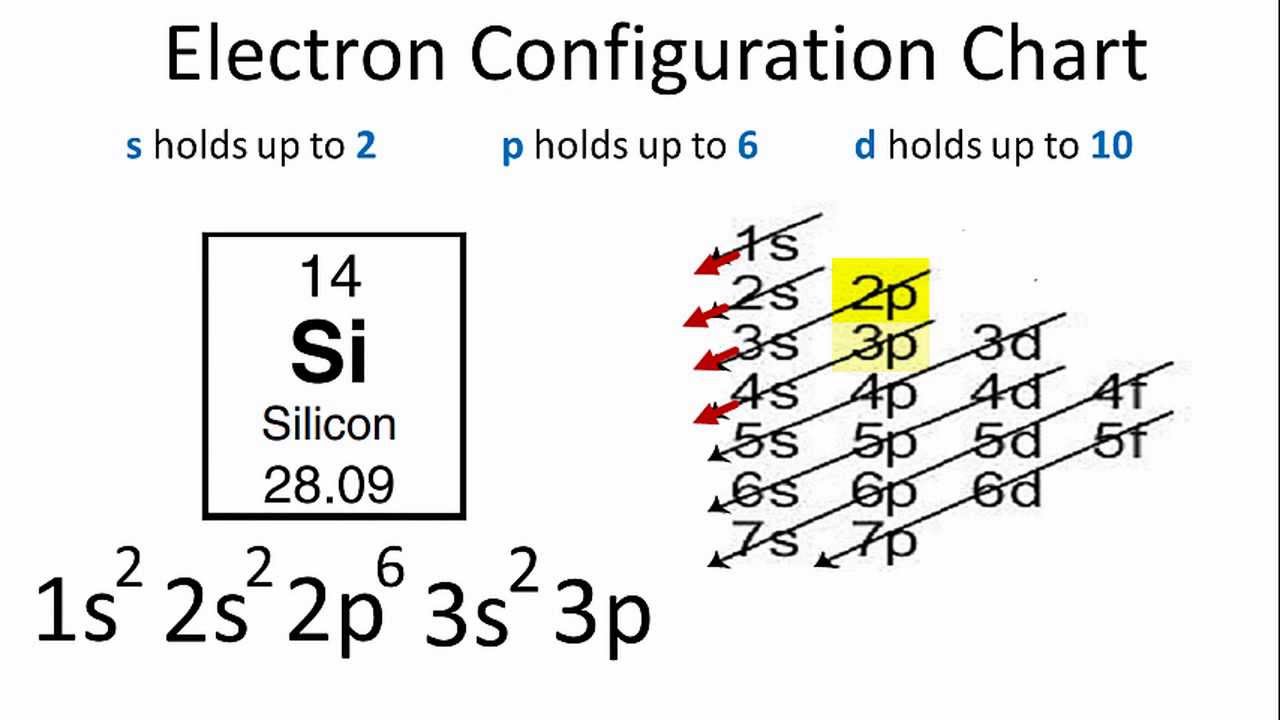
However, it can cause the serious lung disease silicosis if siliceous dust is inhaled. Silicon is important in both animal and plant life. Present in the sun and stars, silicon is the second most abundant element, making up over a quarter of the earth’s crust. Hund’s rule – each orbital should be first filled with one electron before being paired with a second electronĪlso, you should know the number of orbitals in each subshell. Silicon has an electron configuration of 1s 2s 2p. Electron configuration: Ne3s23p2 Oxidation state: 4 Crystal structure: cubic.Pauli exclusion principle – two electrons with the same spin can not occupy the same orbital.The 14 electrons can be distributed amongst the first three atomic shells in a 2,8,4 configuration. Then, the electronic configuration of an element describes how its electrons are distributed in its orbitals. Aufbau principle – electrons are first filled in lowest energy orbital and then in higher energy orbital The number of electrons in K orbit 2 (1) 2 2.Draw orbital diagramīefore drawing the orbital diagram, you should know the three general rules. Electron Configuration of the elements Antimony, Kr5s24d105p Moscovium, Rn7s25f146d107p Argon, Ne3s23p Neodymium, Xe6s24f Arsenic, Ar4s23d104p. Now in the next step, start drawing the orbital diagram for silicon. (For example, Ar4s 2 3d 8 would be entered as Ar4s23d8. Express your answer in condensed form as a series of orbitals.
#SILICON ELECTRON CONFIGURATION HOW TO#
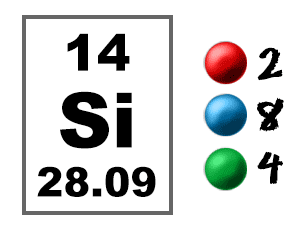
Learn how to find: Silicon electron configuration Give the ground-state electron configuration for silicon (Si) using noble-gas shorthand. The trends of properties of group 14 elements may be largely understood from the valence shell electronic configuration. The electron configuration of silicon is 1s 2 2s 2 2p 6 3s 2 3p 2. Since the atomic number of silicon is 14, the total electrons of silicon are 14. The atomic number of silicon represents the total number of electrons of silicon.


 0 kommentar(er)
0 kommentar(er)
